Why 70 Isopropyl Alcohol Is a Better Disinfectant Than 99 Isopropyl Alcohol When It Come. Why Not Use Higher Isopropyl Alcohol 91 Concentrations.
The presence of water is a crucial factor in destroying or inhibiting the growth of pathogenic microorganisms with isopropyl alcohol.

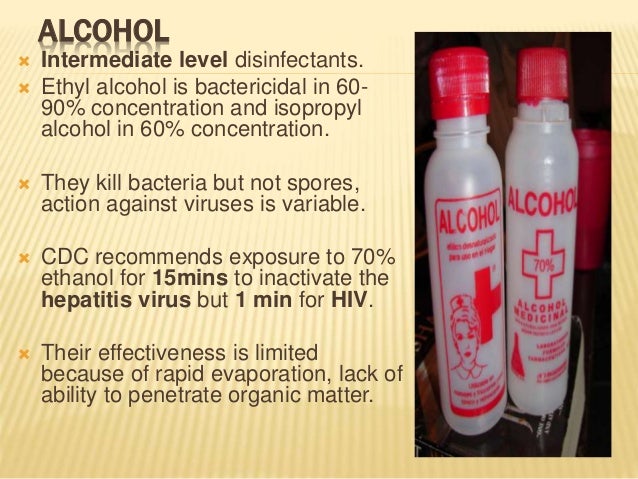
Why is 70 alcohol a better disinfectant than 95 alcohol. But according to microbiology 70 percent alcohol is probably more effective than 91 percent for disinfecting depending on what kind of germs youre trying to kill. 70 IPA solutions penetrate the cell wall more completely which permeates the entire cell coagulates all proteins and therefore the microorganism dies. A 70 alcohol contains more water more dilutedThe water helps the ionization of the alcohol to have more free ions which have the disinfectatnt effect.
70 isopropyl alcohol solution kills microorganisms by dissolving plasma membrane of the cell wall. Why 70 Isopropyl Alcohol Is a Better Disinfectant Than 99 Isopropyl Alcohol. A 70 percent isopropyl alcohol solution.
The important thing is that only 70 solution of isopropyl alcohol acts as a disinfectant killing all surface microorganisms. The theory behind this is that since alcohol is the main active ingredient in the CDC recommended alcohol-based sanitizers more alcohol should automatically mean greater efficacy. Isopropyl alcohol 70 percent or isopropyl alcohol 99 percent diluted to 70 percent with purified water kills organisms by denaturing their proteins.
70 percent of alcohol is ideal to a stronger solution. A bacteria exposed briefly to 95 alcohol experiences a thickening and shrinkage of its cell membrane from which it can recover. Why is 70 percent alcohol a better disinfectant than 95 percent alcohol.
A lower percent-alcohol means theres more water diluting the mix in the bottle. As a disinfectant the higher the concentration of alcohol the less effective it is at killing pathogens. 70 ethanol is also much safer in the air and less likely to affect workers in a facility.
Though ethyl alcohol like methyl alcohol presents a much higher vapor pressure at room temperature than that of IPA about 36 percent the biggest problem with ethyl alcohol is that is a substance regulated by the government. Intriguingly 70 alcohol is a more effective antiseptic than 100 alcohol. Because alcohol causes protein to coagulate on contact a 100 solution coming into contact with a microorganism creates a hardened protein wall around the outside of the organism rather than permeating into its interior.
Heres why a lower-percentage alcohol might be a better weapon against germs. There are two types of ethanol on the market. 70 is preferred for cleaning while 95 is used as a solvent.
It is used to disinfect hands and equipment surface in pharmaceuticals. Water acts as a catalyst and plays a key role in denaturing the proteins of vegetative cell membranes. Thats why 70 is the best concentration to use and it is better than 90.
70 isopropyl alcohol is by far better at killing bacteria and viruses than 90 isopropyl alcohol. Thats a very simplified way to describe the reason but essentially the reason. 70 IPA30 water solution produces less vapor and odor hence decreasing risks of toxic fumes or combustion.
Many think high-percentage alcohol solutions offer greater protection against germs. Pure alcohol coagulates protein in contact. The addition ot the water in 70 alcohol allows water along with alcohol to enter the bacterias cell membrane and destroy the bacteria.
70 isopropyl alcohol maintains key prerequisites for use as a bactericidal in cleanrooms or medical facilities yet in addition to general purposes. Suppose the pure alcohol is poured over a single celled organism. Because some people have been known to drink ethyl alcohol the government requires it be denatured.
In many states its impossible to buy 100 ethanol without a license due to safety requirements. 2 Lipids are more soluble in 70 than 95 so that the oily base that the bacteria stick to is more. Concentrations of alcohol in water and it has been found that 70 works better than 95.
Because you need also water to denature the proteins enough water but not too much not to dilute ethanol.
 70 Percent Of Alcohol Is Ideal To A Stronger Solution Pure Alcohol Coagulates Protein In Contact If 70 Percent Of Alcohol Is Poured To A Single Celled Organism The Diluted Alcohol Also
70 Percent Of Alcohol Is Ideal To A Stronger Solution Pure Alcohol Coagulates Protein In Contact If 70 Percent Of Alcohol Is Poured To A Single Celled Organism The Diluted Alcohol Also
 Why Is 70 Isopropyl Alcohol Ipa A Better Disinfectant Than 99 Isopropanol And What Is Ipa Used For
Why Is 70 Isopropyl Alcohol Ipa A Better Disinfectant Than 99 Isopropanol And What Is Ipa Used For
Why 70 Percent Alcohol Is A Better Disinfectant Than 95 Percent Alcoho Be Moxe Llc
 Why 70 Percent Alcohol Is A Better Disinfectant Than 95 Percent Alcoho Be Moxe Llc
Why 70 Percent Alcohol Is A Better Disinfectant Than 95 Percent Alcoho Be Moxe Llc

 Infection Control 70 Isopropyl Alcohol Isopropyl Alcohol 99 95 Ethanol
Infection Control 70 Isopropyl Alcohol Isopropyl Alcohol 99 95 Ethanol
 Why Is 70 Isopropyl Alcohol Ipa A Better Disinfectant Than 99 Isopropanol And What Is Ipa Used For
Why Is 70 Isopropyl Alcohol Ipa A Better Disinfectant Than 99 Isopropanol And What Is Ipa Used For
 Why 70 Isopropyl Alcohol Ipa Is Used As Disinfectant In Pharmaceuticals Pharmaceutical Guidelines
Why 70 Isopropyl Alcohol Ipa Is Used As Disinfectant In Pharmaceuticals Pharmaceutical Guidelines
 Why 70 Percent Alcohol Is A Better Disinfectant Than 95 Percent Alcoho Be Moxe Llc
Why 70 Percent Alcohol Is A Better Disinfectant Than 95 Percent Alcoho Be Moxe Llc
 Covid 19 Not All Hand Sanitizers Work Against It Here S What You Should Use Health The Jakarta Post
Covid 19 Not All Hand Sanitizers Work Against It Here S What You Should Use Health The Jakarta Post
 Homemade Hand Sanitizer Recipes That Could Help Protect Against Coronavirus Health The Jakarta Post
Homemade Hand Sanitizer Recipes That Could Help Protect Against Coronavirus Health The Jakarta Post
Why Is 70 Alcohols A Better Disinfectant Than 95 Alcohols Quora
Oral And Maxillofacial Surgery Sterilisation And Disinfection

Comments
Post a Comment